BACKGROUND
Colorectal carcinoma (CRC) constitutes aggressive cancers of the colon and rectum, and it is the third most common cancer among men and the second most common among women globally. CRC incidence varies globally, with the highest incidence reported in developed countries.1 However, recent data shows that CRC incidence rates in developing countries, including African countries, are on the rise.2 Several factors have been attributed to the increase in incidence rates, including genetic predisposition, dietary practices such as increased intake of energy-dense foods, urbanization associated with a sedentary lifestyle, and obesity.1,2
CRC in children and young adults has been reported to not be common albeit in a few cases that have been reported. It has been estimated that 1–2 children and/or adolescents are diagnosed with CRC in 1 million people globally. However, the incidence appears to be increasing.3 It is estimated that only 1% of all CRC occur in patients less than 30 years.4 The age-adjusted incidence rate of CRC in the population of adults is said to be 43.7 people per 1 million. A trend of increasing incidence of CRC has been observed in sub-Saharan Africa (SSA).2 Our recent works in northern Tanzania found a higher incidence of CRC in younger individuals, and 90% of the patients presented with advanced disease stages, hence the poor prognosis.5–7 CRCs are generally rare among teens especially in the absence of known risk factors such as a positive family history of CRC, inflammatory bowel disease, familial adenomatous polyposis, or hereditary nonpolyposis colon cancer syndrome.2 The clinical features are generally similar in all ages, but CRC is easily overlooked among the differentials in young patients.2 Other general risk factors for CRC are smoking, obesity, race, diet and old age.
Mucinous carcinomas account for 5-10% of all primary CRCs.1 It is defined by WHO as ‘an adenocarcinoma in which a substantial amount of mucin (>50% of the tumor) is retained within the tumor’. In the past, a myriad of descriptive terms has been used to indicate the mucin-producing phenotype, including colloid carcinoma, gelatinous carcinoma, or degenerative carcinoma. Mucinous adenocarcinomas are clinically, morphologically, and molecularly different from conventional adenocarcinomas, and affect younger individuals, and they are more likely to be associated with Lynch syndrome (LS).8 Mucinous adenocarcinomas are a histological subtype of CRC characterized by secreting extracellular mucin. Mucinous adenocarcinomas are divided into two categories: (i) extracellular subtype; is characterized by tumor cells floating freely in large pools of mucin. The cells that are floating in the mucin often have a bland histologic appearance. (ii) intracellular subtype; is morphologically identical to the signet cell cancer seen in the stomach. The signet cell variant behaves very aggressively, and it has a poor prognosis.8,9 Mucinous adenocarcinomas, including signet cell cancers, account for approximately 10% of colorectal cancers. Compared with non-mucinous CRCs, mucinous carcinomas usually present at a more advanced stage, they have more extensive perirectal spread, they show a greater incidence of lymph node involvement, and they tend to have an overall poorer prognosis.8 Among the mucinous tumors, the extracellular type is much more common than the intracellular, or signet cell type.
Mucinous tumors are characterized by the overexpression of the mucin gene MUC2 and the more frequent ectopic expression of gastric mucin MUC5AC, which is mediated by the transcription factor SOX2. The overexpression of MUC2 is driven by the hypomethylation of the MUC2 promoter, contributing to the mucin-producing phenotype seen in these tumors. This molecular signature is important for understanding the pathogenesis and potential therapeutic targets in mucinous tumors.9 The prognostic value of mucinous histology remains controversial, however, several literatures report they are associated with poor prognosis.8
Mucinous colorectal adenocarcinoma is most commonly found in the proximal colon, where it is frequently diagnosed at more advanced stages, contributing to its poorer prognosis. The larger calibre of the proximal colon allows tumors to grow significantly before symptoms appear. Additionally, mucinous adenocarcinomas are reported to have a poorer response to chemotherapy compared to non-mucinous variants.10 Our case is unusual as the mucinous adenocarcinoma was located distally, highlighting a rare presentation of this pathology. MRI is usually helpful in detecting this unusual CRC as they characterized by a low signal intensity in T1-weighted images and a significant hyperintense signal in T2-weighted images. Non-mucinous colorectal adenocarcinoma displays an intermediate signal intensity in T2-weighted images on the other hand. On CT scan, they are typically distinguished by a thickened intestinal wall, a thickened gut mucosa, and the presence of low-density cystic lesions. Additionally, these tumors show non-significant enhancement in the arterial phase compared to normal muscle, aiding in their identification and differentiation from other types of CRC.10
LS is characterized by predisposition to colorectal, endometrial, and other cancers and is caused by inherited pathogenic variants affecting the DNA mismatch repair (MMR) genes MLH1, MSH2, MSH6, and PMS2. LS1 and LS2 are both associated with MMR malfunction and microsatellite instability.11 The prognosis is generally poor in the pediatric group, as reported in the literature, however, little has been published.8 Herein, we present an uncommon case of advanced mucinous CRC in a teenager.
CASE PRESENTATION
A 19-year-old male patient was referred to our facility from a primary health care facility with a presentation of abdominal distension for 3 weeks accompanied by early satiety and bloody diarrhea. The symptoms were progressive. These symptoms were preceded by a 5-month history of diarrhea, intermittent episodes of constipation, and unintentional weight loss. During the illness, he tried various over-the-counter medications without relief. His past medical history was otherwise unremarkable. There was no familial history of gastrointestinal/breast/endometrial/prostate cancers, and he denied a history of consuming alcohol and tobacco.
On physical examination, he was conscious, afebrile, mildly pale, not jaundiced, with bilateral pitting lower limb edema. He was wasted and undernourished as most of his bonny prominences were appreciated. The vital signs were within normal limits. His abdomen was grossly distended, non-tender, no organomegaly, hypertympanic on percussion, and exaggerated bowel sounds were appreciated on auscultation. On rectal examination, a hard mass easily bleeding and completely obstructing the distal rectum was felt hence could not go above it and was approximately 6cm from the anal verge. A respiratory examination revealed reduced air entry on the left side with a dull percussion note.
Investigations
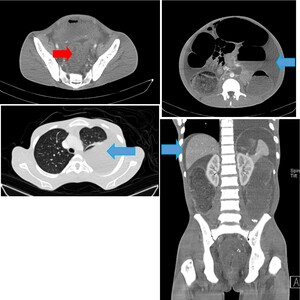
His complete blood count showed a hemoglobin of 9.4 g/dl (microcytic and hypochromic), leucocyte count of 9.22 × 109/l and a platelet count of 142×109/L. A plain chest x-ray revealed approximately 50% left pleural effusion (Figure 1), and abdominal-pelvic ultrasound concluded ascites, an ill-defined heterogenous pelvic mass with mild ascites. A contrasted CT-scan of the chest and abdomen showed symmetrical mural wall thickening measuring 4.2 cm of the sigmoid, upper, mid, and distal rectum measuring 16 cm in length, suggestive of a rectosigmoid tumor. The tumor was causing large bowel obstruction with ascites, and left sided pleural effusion (Figure 2).
Management
He was transfused with one unit of whole blood (450 mL), and a left-sided thoracostomy tube was placed to drain the pleural effusion. He was then taken for an emergency colostomy to relieve the obstruction. The abdomen was opened through a upper midline incision. Intraoperatively, approximately 2 liters of hemorrhagic ascites were found. A hard, irregular pelvic mass, white in color, was identified, extending from the rectum to the anal canal and causing obstruction of the colon. Multiple enlarged lymph nodes were noted on the bowel mesentery, extending to its root, as well as in the paraaortic and celiac regions. Peritoneal metastasis was observed on the mesentery, omentum, liver, stomach, and anterior abdominal wall, consistent with Stage IV disease (cT4NxM1). A double-barrel mid-transverse colostomy was created, and a biopsy was taken.
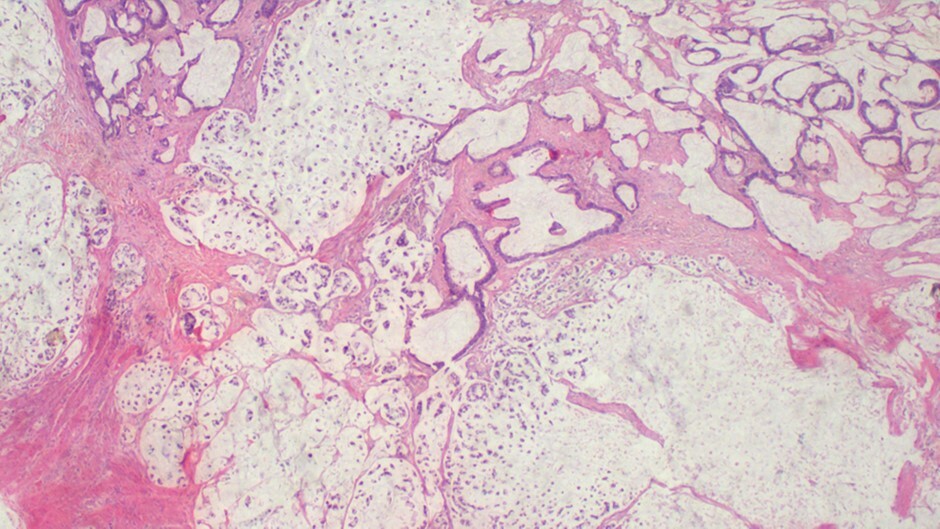
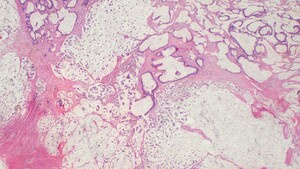
Histopathology revealed an infiltrative tumor composed of atypical glandular structures, with strips of tumor cells floating in large extracellular mucin lakes consistent with mucinous adenocarcinoma with omental metastasis, pT4NxM1 (Figure 3).
Outcome and follow-up
His post-operative recovery was not promising, was managed in the surgical ICU with intravenous fluids, antibiotics, analgesia and 450 mL of whole blood transfusion as per local protocol, and unfortunately, he succumbed nine days after surgery. The next of keen was counseled prior to surgery on the advancement of the disease and poor outcome. First degree relatives were counseled and screened negative by CEA and colonoscopy.
DISCUSSION
The delay in diagnosing colorectal cancer (CRC) in young patients, as demonstrated in the index case, is concerning in terms of improving prognosis.5 The absence of clear clinical features commonly associated with this malignancy is a significant factor contributing to poor outcomes. Consequently, there is a need for CRC screening in children and young adults, especially those with a family history of the disease.6 Furthermore, clinicians should ensure that all patients, including children and young adults showing any signs or symptoms of CRC, are thoroughly evaluated and closely followed to detect and treat the disease at its early stages.
CRC incidence is increasing globally among the younger generation, with a high fatality rate, however, it’s largely preventable. Screening can reduce mortality rates, especially among young people, aged under 50 years. Implementing proven screening measures and developing innovative risk assessment methods can help reduce CRC risk, especially in developing countries.1 In recent decades, there has been a steady decline in CRC incidence among the older population, which contrasts sharply with the rising incidence in young adults. CRC is increasingly recognized as an indicator of epidemiological change and socioeconomic shifts. The incidence and mortality rates of CRC are rising in low- and middle-income countries, in stark contrast to the trends observed in developed nations. For example, data from the Kampala Cancer Registry highlight a dramatic increase in CRC rates. Specifically, in Tanzania, there has been a six-fold rise in the incidence of CRC.12 The authors found that patients presented at late stage of CRC, secondly there were younger patients diagnosed with CRC and thirdly there was a low rate of diagnostic colonoscopy on CRC patients. This not only shows a shift in the presentation of age but also a shift towards rise in non-communicable diseases in developing regions.12
Early-onset colorectal cancer (EOCRC) has now become the second most common cancer and the third leading cause of cancer-related deaths in younger populations. Similar upward trends are seen in Europe and North America, with a notable rise in distal colon and rectal cancers. A detailed analysis from 20 European countries revealed an annual increase in CRC incidence by 7.9% in individuals aged 20–29 years, 4.9% among those aged 30–39, and 1.6% in the 40–49 age group from 2004 to 2016. This data underscores the urgency of addressing the rising burden of CRC in younger populations.13
Early-onset CRC is a highly heterogeneous disease, with hereditary genetic syndromes accounting for only a small proportion of cases, while the majority are classified as sporadic.14 Mortality rates are higher in younger CRC patients compared to their elderly counterparts. This trend may be explained by two key factors: earlier exposure to known risk factors in the younger generation compared to previous ones, and the fact that neoplasms appearing at a younger age are often biologically more aggressive.15
In addition to well-known risk factors such as inflammatory bowel disease and hereditary syndromes linked to CRC, other factors may contribute to the rising incidence of early-onset colorectal cancer. Lifestyle changes, including increased alcohol consumption, a sedentary lifestyle, high red meat intake, obesity, and diabetes mellitus, may partially explain this trend. However, current epidemiologic data are insufficient to establish a definitive correlation between these factors and early-onset CRC.16
Various screening tests are available for CRC, each with its own advantages and disadvantages, and the quality of evidence supporting these tests varies. Common methods include guaiac-based fecal occult blood tests (FOBTs), fecal immunochemical tests (FITs), flexible sigmoidoscopy, and colonoscopy, among others.17,18 While these screening methods are valuable, they have limitations, and many eligible individuals remain unscreened. Although novel screening techniques may help in early CRC detection and risk factor modification, significant benefits can still be achieved by optimizing existing screening methods. Chalya et al. highlight that CRC is rising in sub-Saharan Africa, attributed to urbanization and lifestyle changes. In contrast, CRC incidence is declining in developed countries due to improved outcomes from early detection, widespread screening of high-risk populations, and advancements in diagnostic tools and treatment options.19 The authors note that CRC in African populations often present at a younger age and at a more advanced stage, leading to poorer prognosis, as illustrated by our case.
CRC in younger patients is often associated with inherited syndromes such as hereditary nonpolyposis colorectal cancer (Lynch syndrome), inflammatory bowel disease, familial adenomatous polyposis (FAP), and MUTYH polyposis.20 This highlights age as an important factor for identifying familial and genetic predispositions.18 Due to limited resources, we were unable to conduct genetic screening on our patient, and genetic predisposition could not be assessed. Several family history criteria can suggest Lynch syndrome, notably the Amsterdam II Criteria and Revised Bethesda Guidelines. These guidelines are helpful in identifying families likely to have Lynch syndrome.
These include: (i) multiple family members with colorectal cancer, particularly if diagnosed under age 50. (ii) presence of other Lynch syndrome-associated cancers in the family, such as endometrial, ovarian, or gastric cancer. (ii) cancers occurring across multiple generations. (iii) synchronous or metachronous colorectal cancers in one individual.11 However, the morphological features observed in our case—such as infiltrating lymphocytes, peritumoral lymphocytes, the absence of dirty necrosis, and mucinous features—strongly suggest a microsatellite instability-high (MSI-H) etiology, likely linked to LS. We recommend clinicians perform genetic testing on young CRC patients whenever feasible. Colonoscopy would have been valuable to rule out conditions like FAP and MUTYH polyposis, which typically present with multiple colonic polyps. However, due to the patient’s unstable condition, colonoscopy could not be performed. In the index case, the teenager was diagnosed with advanced colorectal mucinous adenocarcinoma, aligning with findings by O. K. Ibrahim et al., who reported that colorectal cancers in younger populations are frequently either mucin-secreting or signet-ring cell carcinomas. They present at advanced stages, and are associated with a poor prognosis, and this is due to the marked delay in diagnosis.21 A similar case presented by Akpa et al. of mucinous colon cancer in a 11-year-old female, and they state that these CRC in younger generation is poorly differentiated hence more aggressive and less chemo-sensitive, suggestive there may be differences in the biological pathogenesis of these tumors compared to that of adults.4
There are various advances in immunotherapy, however, its implications to CRC patients remains uncertain as there are various on-going studies. Current immunotherapy approaches for CRC including cancer vaccines such as autologous vaccine, peptide vaccine, viral vector and dendritic cell (DC). Other immunologic therapies in trials are oncolytic virus therapy, immune checkpoint inhibitors therapy and immune modulators such as IDO1-inhibitors and anti-OX40 agonist therapy.22
Conventional cancer treatment strategies widely used today include surgical tumor resection, often followed by radiotherapy with x-rays and/or chemotherapy. Surgery is most effective during the early stages of disease progression. However, radiation and chemotherapy pose risks, such as damage to healthy tissues and growing cells. Additionally, drug resistance remains a significant challenge in chemotherapy treatments. Targeted drug therapy offers a solution by specifically attacking cancer cells while minimizing damage to normal, healthy cells. This approach exploits the unique programming that distinguishes cancer cells from healthy ones. Widely used targeted agents include monoclonal antibodies and small molecule inhibitors.23
A limitation of our case study is that we were unable to confirm a diagnosis of LS due to the unavailability of germline mutation testing in our setting. Similarly, although immunohistochemical testing for mismatch repair (MMR) proteins (MLH1, MSH2, PMS2, MSH6) is a reliable method for diagnosing Lynch syndrome, this was also not feasible due to the patient’s financial constraints. Our case study, along with similar reports of early-onset CRC, highlights the need for improved CRC screening and the development of genetic counseling services to help prevent advanced CRC in at-risk family members.
CONCLUSION
Colorectal cancer (CRC) in patients under 40 years of age is relatively rare, with mucinous adenocarcinoma representing a particularly uncommon and aggressive subtype. This demographic often experiences challenges such as late presentation, delayed diagnosis, and a lack of definitive clinical symptoms, which frequently lead to poorer outcomes. To mitigate these issues, early and proactive screening is essential, especially for individuals with a family history of CRC. Physicians must maintain a high level of vigilance and conduct comprehensive evaluations for young patients exhibiting colorectal symptoms. By prioritizing early detection and timely treatment, healthcare providers can significantly improve prognosis and patient outcomes.
CRC and its management pose significant diagnostic and therapeutic challenges in resource-limited countries like Tanzania. The late presentation of CRC and the absence of adequate screening programs contribute to high morbidity and mortality rates in these developing regions. To address these issues, stakeholders should focus on improving population education, training clinicians at the primary health level, implementing screening programs for high-risk individuals, and introducing cost-effective treatment options. Additionally, enhancing follow-up techniques is crucial for better management and outcomes in CRC patients.
CONSENT
Written informed consent was obtained from a legal authorized representative for publication for this case report; additionally, accompanying images have been censored to ensure patient confidentiality. A copy of the consent is available on record.
ETHICAL APPROVAL
Ethics Committee approval was not required for this case report. The surgical procedure was conducted in accordance with the principles of the Declaration of Helsinki.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Dr. Gregory Goodluck for his assistance in retrieving and summarizing the patient clinical data for this case report.
.jpg)
.jpg)