Background
Lung carcinoids are a subset of neuroendocrine tumors originating from the neuroendocrine cells in the bronchopulmonary tract. They account for 1%–2% of all lung cancers but represent 30% of all neuroendocrine tumors.1 These are slow-growing tumors that are subclassified into typical and atypical carcinoids based histologic markers. Typical carcinoids, also known as well-differentiated neuroendocrine tumors, account for 80.4% of cases and have the best prognosis. The mean age at diagnosis is around 45 years, affecting females slightly more compared to males. The prevalence of lung carcinoids seems to be increasing, which may be attributed to advancements in diagnostic techniques rather than environmental factors.2
All pulmonary carcinoid tumors should be treated as malignancies. Surgery is the only curative approach, and treatment typically involves anatomic resection to achieve negative margins and lymph node dissection. In some cases, sleeve resection or other complex airway reconstruction may be required. In our patient’s case, the very proximal position of the tumor and delay in presentation resulting in chronic lung injury required complex reconstruction on mechanical support for oxygenation.
Case Presentation
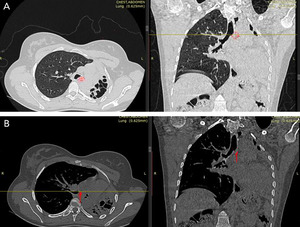
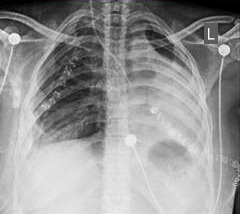
A 27-year-old female patient presented with chest pain and an intermittent productive cough with bloody sputum for many months. She had no history of pulmonary tuberculosis and had never smoked. Chest examination findings indicated signs of volume loss and decreased breath sounds over the left side. Other system evaluations were unremarkable. Chest X-ray and computerized tomography (CT scan) revealed a completely obstructing mass in the proximal left mainstem bronchus (Figure 1A and B). Bronchoscopic evaluation showed a red, fleshy mass in the left main bronchus. A tissue biopsy of the mass confirmed the diagnosis of a left bronchial carcinoid tumor (Figure 2).
The very proximal location of the tumor and chronic consolidation of the left lung complicated surgical management. Surgical resection with a left carinal pneumonectomy was planned. Preoperatively, an electrocardiogram (ECG) and echocardiogram were within normal limits. A right thoracotomy approach with intraoperative veno-arterial extracorporeal membrane oxygenation (ECMO) support was planned in order to provide access to the carina and distal trachea. The patient underwent a left carinal pneumonectomy, starting with a right fourth interspace thoracotomy and exposure of the tracheobronchial tree to assess the extent of the tumor. The patient was then placed on ECMO through femoral approach. As a dedicated ECMO circuit was not available, a Soren 4 bypass system with a Medtronic Affinity Fusion oxygenator was used. The venous reservoir was excluded from the circuit to reduce thrombotic risk. Activated clotting time (ACT) was kept approximately 150 as the circuit was not fully heparin bonded. The trachea was divided 1 cm above the carina, the right main bronchus was divided 0.5 cm below carina, and then an anastomosis was performed between the right main bronchus and the trachea with 4-0 PDS suture. After confirming the integrity of the anastomosis, the patient was weaned from ECMO and decannulated. ACT was normalized with protamine. Following closure of the right thoracotomy, the patient was repositioned and a left thoracotomy and then a left pneumonectomy was performed. Although there were dense adhesions, resection was uneventful and the specimen was sent for pathologic review.
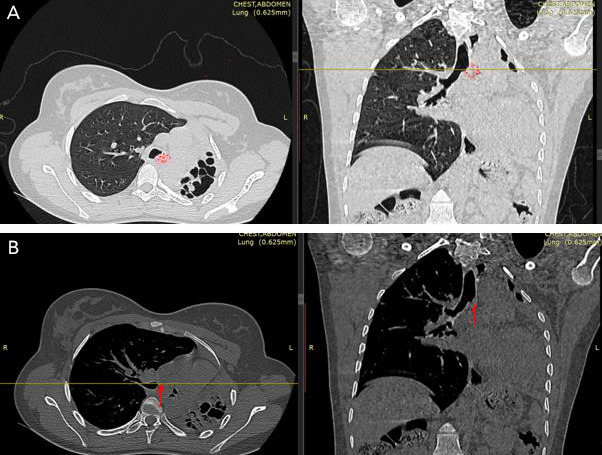
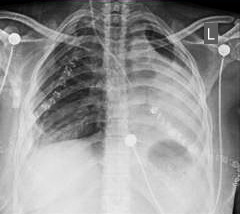
Postoperatively, the patient was transferred to the intensive care unit and received oxygen support, analgesics, prophylactic antibiotics, and daily chest radiographs. She experienced a smooth recovery and was subsequently transferred to the surgical ward. The left chest tube was removed on postoperative day 1, and by postoperative day 2, the fluid level was rising adequately in the left chest cavity. The right chest tube output was minimal by postoperative day 2. A chest radiograph was performed demonstrating a well-expanded right lung with no evidence of pleural collection, allowing for the removal of the chest tube (Figure 3). She was discharged on day 7 in good condition.
Histopathological examination of the excised tumor and left lung confirmed a typical carcinoid/neuroendocrine tumor, grade 1 with 1–2 mitoses/10HPF, no lymphovascular invasion, and negative margins. No lymph nodes contained tumors. The pathologic stage classification3 was pT1bN0. Immunohistochemistry results were positive for synaptophysin, chromogranin, and Ki-67.
Discussion
The overall incidence of carcinoid tumors is estimated to be 1 to 2 cases per 100,000 individuals in the United States, a rate similar to that in England, Scotland, Spain, Italy, and Japan. This incidence is rising, potentially due to improved diagnostic methods. The incidence in the College of Surgeons of East, Central and Southern Africa (COSECSA) region is unknown. The average 5-year survival rate for typical carcinoids is 93%, while for atypical carcinoids it is 69%.4
Up to half of patients with lung carcinoids are diagnosed incidentally during routine chest X-rays or CT scans. Clinically, lung carcinoids often present with symptoms such as cough, hemoptysis, wheezing, dyspnea, recurrent pneumonia, and chest pain. Carcinoid syndrome occurs in 2%–12% of patients with lung carcinoids, particularly when liver metastases are present. Metastatic disease is found in 5%–20% of patients with typical carcinoids and up to 70% of those with atypical carcinoids, with metastases commonly affecting regional lymph nodes and other distant sites.5 Bronchoscopy is a critical tool for obtaining tissue samples, especially in centrally-located carcinoids. Flexible bronchoscopy is generally preferred, but rigid bronchoscopy may be used in patients at high risk for bleeding or when larger biopsy samples are needed. Endobronchial ultrasound is the best tool to evaluate lymph node metastasis. Somatostatin receptor scintigraphy is recommended in selected cases, particularly those with ectopic hormone production, to detect metastatic disease. Positron emission tomography can be useful in more aggressive cases for tumor detection, staging, and evaluation of metastases.6
Surgical removal is the preferred treatment for pulmonary carcinoids. The choice of procedure depends on several factors: tumor type (typical/atypical), presence of lymph node metastases, surrounding tumorlets, and the patient’s age and lung function. Peripheral tumors can be removed by wedge resection, segmentectomy, or lobectomy/bilobectomy. Central tumors can be treated with bronchotomy, bronchoplasty, sleeve resection, or carinal pneumonectomy. Left carinal pneumonectomy is a highly complex surgical procedure involving the resection of the carina en bloc with the removal of the left lung.7 This is typically indicated for tumors involving or near the carina. Cross-table ventilation of the left lung with a long wire re-enforced single lumen endotracheal tube is often used for support during dissection of the carina, and tracheal to right mainstem anastomosis. In this patient, chronic consolidation and damage to the lung prohibited the option of cross field single lung ventilation of the left lung. Cross-table ventilation of the right lung was also considered. To ensure adequate margin of resection, the tracheal to right mainstem anastomosis was performed a cartilaginous ring above the right upper lobe takeoff. As opposed to tracheal resection and re-anastomosis, proximity of the anastomosis to lung parenchyma of the hyperexpanded lung with shift of the mediastinum toward the left makes visualization for anastomosis difficult with a ventilating lung. Mechanical support was the optimal means to facilitate anastomosis. ECMO was preferred over standard cardiopulmonary bypass as less anticoagulation is needed given the bilateral thoracotomy exposure and left pleural adhesiolysis and empty left pleural cavity at the conclusion of the procedure. Cardiopulmonary bypass is a well-established process at King Faisal Hospital, and we were able to modify an existing circuit by excluding the reservoir to mimic an ECMO circuit that does not require full systemic anticoagulation. Veno-venous ECMO is a viable and perhaps preferred method of support since hemodynamics should not be impaired intraoperatively, but this method had not been previously used at our institution. Veno-venous ECMO could be initiated with bilateral femoral access or internal jugular and femoral access. Insertion and maintenance of cannula position can be complex when repositioning a patient for thoracotomy. For this reason, we elected for veno-arterial ECMO through the right groin during the right thoracotomy for parts of the dissection of the carina, division of the airways, and re-anastomosis. Competitive flow from healthy left ventricular ejection can result in a north/south phenomenon, leading to differential saturation. Maintaining adequate flows equaling cardiac output and consideration of negative inotropy like beta blockade can minimize the risk of hypoxic events. Monitoring of the right upper extremity pulse oximetry was performed, as cerebral oxygenation could not be monitored.
The anastomosis involves reattaching the trachea to the right mainstem bronchus, and this should be done meticulously to reduce tension and promote healing. Muscle or mediastinal tissue buttressing can be done to protect the anastomosis. Anastomotic complications, such as dehiscence and stenosis, are significant causes of morbidity and mortality. Early extubation in the operating room is optimal to reduce the risk of ventilator-associated complications. Operative mortality rates for carinal pneumonectomy range from 6%–12%, with morbidity including complications such as anastomotic issues and recurrent laryngeal nerve damage. The British Thoracic Society and the Society of Cardiothoracic Surgeons of Great Britain and Ireland note that while the procedure carries a high operative risk, it can be justified by the potential for long-term survival in selected cases.8
Conclusion
This case report highlights the importance of a comprehensive diagnostic and therapeutic approach in the management of bronchial carcinoid tumors. Early and accurate diagnosis, facilitated by advanced imaging and bronchoscopy may have prevented chronic injury of the left lung and provided alternative operative strategies to ECMO support. However, the location of the tumor required carinal pneumonectomy for curative resection. This case illustrates the complexity of surgical intervention, including the use of ECMO and meticulous postoperative care. Histopathological evaluation confirmed the typical carcinoid nature of the tumor, which has a favorable prognosis when managed appropriately. This report emphasizes the need for a multidisciplinary team to ensure optimal patient outcomes and supports the ongoing advancement in the COSECSA region of diagnostic and surgical techniques in the treatment of bronchial carcinoid tumors.
Ethical Approval/Informed Consent
Formal ethical approval was not required for this case report as per institutional guidelines/local regulations for retrospective reporting of a single clinical case. Written, informed consent was obtained from the patient for publication of this case report and accompanying images.
Data Availability
Not applicable
Conflict of Interest
All authors declare no conflicts of interest.
Funding
None.