Introduction
Volvulus is a term derived from the Latin word volvere, which means to rotate.1 When referring to the colon, it describes the colon turning around its mesentery, which usually occurs in the sigmoid colon and ultimately leads to ischemia followed by bowel gangrene.2
Sigmoid volvulus is the most common form of volvuli.3,4 The incidence of sigmoid volvulus, as well as other types of volvuli, varies geographically. Sigmoid volvulus is common in areas such as the “Volvus belt,” which is an endemic area comprised of Africa, South America, Eastern Europe, and the Middle East,5,6 but it is rare in other areas, such as North America, Western Europe, Australia, and Japan.7
In countries within “the volvulus belt,” sigmoid volvulus is more common in young men, occurring from the fourth decade onward with a male to female ratio of 4 to 1,7 representing 75% higher prevalence in males than females.6 Sigmoid volvulus is the third leading cause of large bowel obstructions worldwide after colonic carcinoma and diverticular disease, and it accounts for more than half of all large bowel obstructions in Eastern Europe, India, and Africa.8
Surgical management of sigmoid volvulus, such as sigmoid resection, can be performed for various reasons. Predominantly among these reasons are acute emergencies9 and sigmoid volvulus with or without peritonitis.8,10,11 Sigmoid resection is followed by either primary anastomosis or Hartmann’s procedure.
Sigmoid resection followed by primary anastomosis consists of resecting the torsed or gangrenous sigmoid segment followed by bowel anastomosis, where the proximal and distal segments are joined to restore bowel continuity. This procedure is performed when the sigmoid is viable and when the general conditions of a patient are favorable.8 Hartmann’s procedure consists of removing a torsed and gangrenous segment of the bowel. Then, the proximal end of the bowel is brought out to the anterior abdominal wall skin to form a stoma for diverting bowel contents. This procedure is performed when patients’ conditions are not favorable or when the sigmoid volvulus is gangrenous or perforated with peritonitis.11
Different studies show that there is no difference between the two procedures in terms of postoperative complications and mortality.8,11 However, a single stage, which involves resection and primary anastomosis, is associated with high mortality in patients with poor general conditions.12,13 A study by Ooko revealed that performing primary anastomosis in gangrenous sigmoid volvulus is safe.14
Given the significant clinical implications and the varying practice patterns globally, a systematic review and meta-analysis comparing the sigmoid resection with primary anastomosis and Hartmann’s procedure for sigmoid volvulus is warranted. This study aims to synthesize the available evidence to provide a comprehensive comparison of the outcomes associated with each procedure, including perioperative morbidity and mortality.
Methods
The methodology of this systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement. The 2020 PRISMA statement is a 27-item checklist.15
The study protocol was registered under PROSPERO CRD42023434923.
Information sources
The PubMed, Google Scholar, Cochrane Library, Africa Journal Line, SCOPUS, and EMBASE databases were searched for this study.
Search strategy
A standardized search strategy was used in PubMed, which was subsequently modified according to each specific database to obtain the most relevant results. Our basic search strategy was based on the following research question formulation (i.e., PICO): (P-population) patients who underwent emergency surgical treatment for confirmed sigmoid volvulus either viable or gangrenous; (I-Intervention) resection with primary anastomosis; (C-Comparison) the Hartmann procedure; (O-Outcome) the results compared were anastomotic leakage, hospital stay, cost, morbidity, and mortality.
The following combination of MeSH terms was used: “intestinal volvulus”; “sigmoid diseases/surgery”; “anastomosis, surgical”; “colon, sigmoid/surgery”; “anastomosis, surgical/complications”; “anastomosis, surgical/mortality”; “anatomical leak”; “anastomotic leak/statistics and numerical data”.
The combinations of key words used were: “sigmoid volvulus”, “sigmoid resection”, “sigmoid volvulus surgery”, “primary anastomosis”, “primary resection and anastomosis”, “Hartmann’s procedure”, “Hartmann’s colostomy”, “anastomotic leak”, “anastomotic leakage”, “morbidity”, “mortality”, “hospital stay”, “treatment outcome”, and “anastomotic wound infection”.
A manual search of the references to identified articles and relevant reviews was also conducted.
The search was limited to the English language, and there was no time limitation. The search was completed August 2023.
Selection process
Two reviewers (JLM, TC) independently assessed the titles and abstracts of all identified articles. The decisions of the two reviewers were recorded individually (select or reject) in the Endnote file and then compared. Any disagreements between the two reviewers were resolved by a third reviewer (MG).
The two reviewers evaluated the full texts of all potentially eligible papers and decided whether to include or exclude each study according to the inclusion criteria. Any study that did not fulfill all the criteria was excluded, and its bibliographical details were recorded with the reason for exclusion being as follows: “The findings of the search process are illustrated according to the PRISMA 2020 statement to provide optimal clarity and transparency.”
Eligibility criteria
Inclusion: This review includes observational studies; case‒control, cohort, and cross section studies; and randomized clinical trials.
Exclusion: Articles that do not compare two techniques, compare surgical techniques and laparoscopic techniques, or compare conservative and surgical management were excluded. Case reports and studies that do not include at least 10 patients were excluded.
Data collection process
The bibliographical details of all the retrieved articles were imported into Covidence, and duplicate records resulting from the various database searches were removed.
Covidence was used for title, abstract, and full-text screening, and the article was subsequently extracted and transferred to SPSS Version 29.
Data items
The primary outcome was the overall in-hospital mortality rate, and the secondary outcomes were anastomotic leakage, intra-abdominal collection and infection, reintervention, intensive care admission, surgical site infection, and hospital stay. These outcomes were selected based on their importance and frequency across the studies.
Data were collected on the author, year of publication, study characteristics (design, study population, mean age, sex), participants (adult patients with non- and gangrenous sigmoid volvulus), intervention characteristics (resection primary anastomosis and comparator via the Hartman procedure), results (P value, odds ratio, and risk ratio), and outcomes (mortality, anastomotic leakage, intra-abdominal collection, number of reinterventions performed, intensive care unit (ICU) admission, abdominal wound infection, hospital stay).
Study risk of bias assessment
The JBI (Joanna Briggs Institute, Faculty of Health and Medical Sciences, University of Adelaide, Australia) critical appraisal tools were used to evaluate the internal and external validity of included studies to reduce the risk of bias. The JBI critical appraisal tool for randomized controlled trials consists of 13 items evaluated as yes, no, unclear, or NA, with overall appraisals of include, exclude, or seek further information. The JBI appraisal tools for case-control studies, cohort studies, and cross-sectional studies consist of 10,11 and 8 items, respectively, with overall appraisals of include, exclude, or seek further information.
The mean score of two authors who evaluated the studies was used for the final decision regarding study inclusion in the meta-analysis.
Two reviewers worked independently and applied the tool to each included study, recording supporting information and justifications of risk of bias judgements in each domain. Any discrepancies in risk of bias judgements or justifications for judgments were resolved through discussion to reach consensus between two review authors with a third review author acting as an arbiter if necessary.
Effect measures
We calculated the odds ratio (OR) and standard error (SE) for binary outcomes. Standardized mean differences, standard deviations, and 95% confidence intervals were used for continuous data.
Synthesis methods
Population, intervention, comparator (Hartmann’s procedure), and outcome (in hospital mortality, surgical site infection) were analyzed.
Relevant data extracted from the studies included were imported into Microsoft Excel and subsequently into SPSS software for analyses of pooled estimates of outcome measures and subgroup analyses.
Considering the variation in true effect sizes across study subjects, Der-Simonian‒Laird’s random effects model was used for the analysis at the 95% confidence level. Variation in study outcomes between studies (heterogeneity) was assessed via Cochran’s Q test, which is linked to the I² statistic: 0–40% (limited), 40–60% (moderate), 60–80% (substantial), and 80–100% (considerable).
Publication bias was evaluated by using funnel plots for visual evaluation of publications and Egger’s tests as a statistical method. A statistical test with a P value above 0.05 (one-tailed) indicated no considerable publication bias.
Results
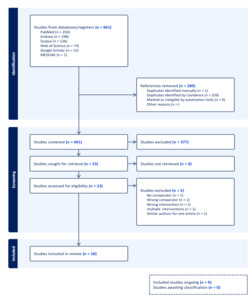
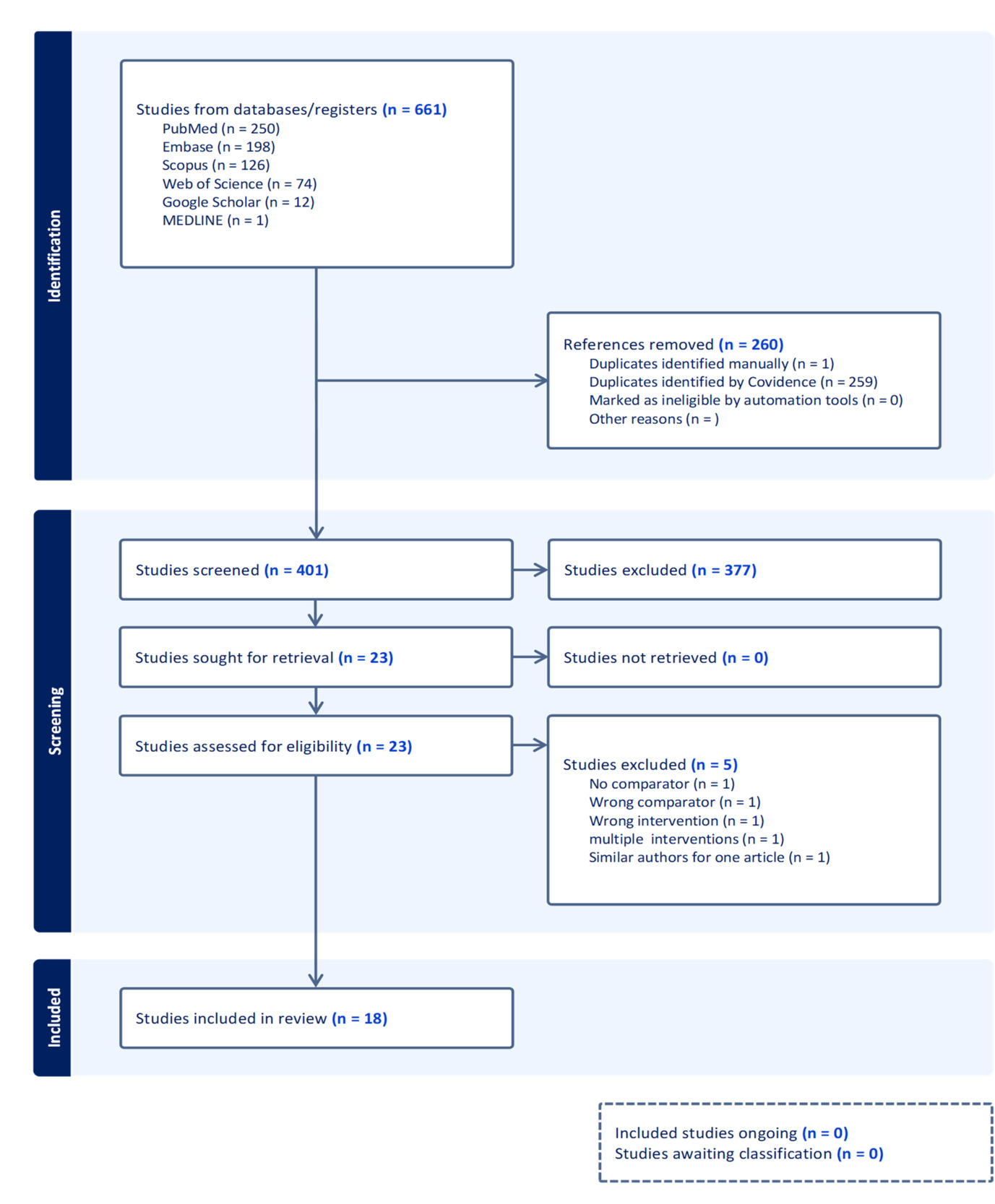
Figure 1 shows a PRISMA flow diagram from Covidence describing the rigorous screening of 661 studies, where duplicates were removed following title, abstract, and full-text screening. Our study included a total of 18 studies: 1 prospective clinical trial and 17 observational studies (3 cross-sectional and 14 cohort) with a population of 4473 patients. The search was performed across different databases (PubMed, Embase, Scopus, Web of Science, and Google Scholar). Manually searched studies were considered similarly to the studies identified via Google Scholar after risk of bias assessment using JBI checklist for clinical trials, cohort, and cross-sectional studies. Only 12 studies were included in the meta-analysis.
Demographics and clinical characteristics
The demographics and clinical characteristics for this study are described in Table 1. There were 2853 males (63.78%) and 1620 females (36.22%). The mean age was 54.11 years (the standard deviation was ± 9.25 years), which was derived from the mean age across the researched studies, not from primary study data.
According to sigmoid viability, 2911 cases of viable sigmoid (65.07%) and 904 cases of gangrenous sigmoid volvulus (20.21%) from the studies that recorded bowel movement restoration after sigmoid resection and according to sigmoid volvulus viability.
Overall in-hospital mortality
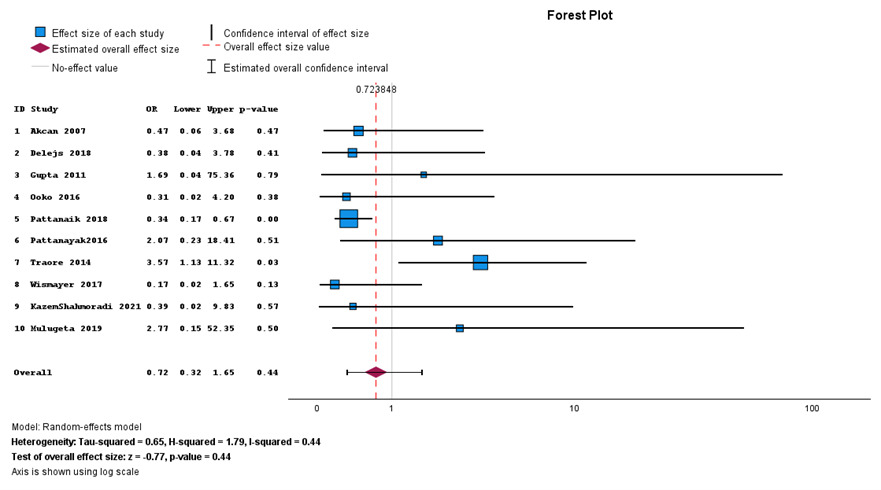
Overall, in-hospital mortality was not statistically different between primary anastomosis and Hartman’s procedure (P value of 0.44, 95% CI = 0.32–1.65) as shown in Figure 2. Most studies are on the left side of the no-effect line.
In further subgroup analysis, there was no preference between primary anastomosis and Hartmann’s procedure for viable and gangrenous patients when analyzed separately.
Mortality in primary anastomosis and Hartman’s procedure for viable sigmoid volvulus
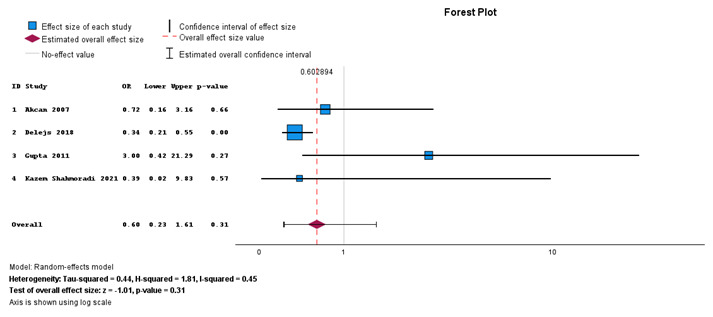
Figure 3 shows the forest plot describing mortality in primary anastomosis and Hartmann’s procedure for viable sigmoid volvulus. There is no statistically significant difference in mortality between the two procedures performed for viable sigmoid volvulus (P value 0.31, 95% CI = 0.23–1.61). There is moderate heterogeneity with I2 = 45%.
Mortality in primary anastomosis and Hartman’s procedure for gangrenous sigmoid volvulus
In Figure 4, estimated overall effect size (diamond shape) is on the primary anastomosis side indicating that mortality is higher in primary anastomosis than in Hartmann’s procedure, but difference was not statistically significant (P = 023). There is limited heterogeneity with I2 = 21%.
Secondary outcome
In an analysis of secondary outcomes, only surgical site infection was uniformly reported and included as a secondary outcome.
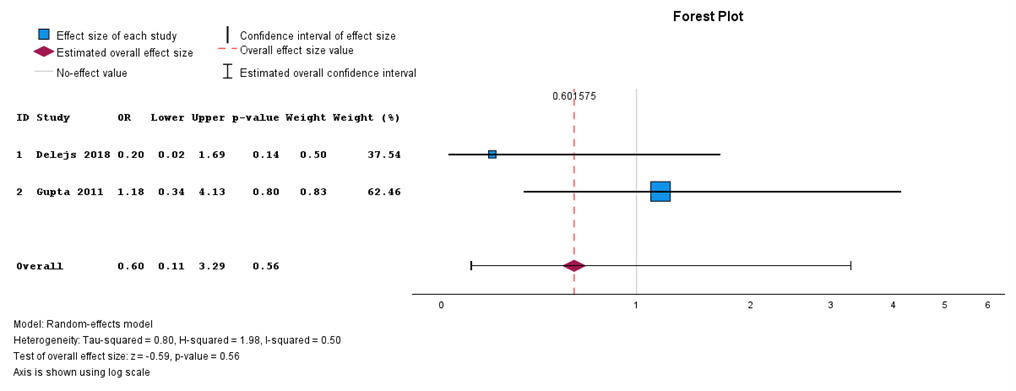
Overall surgical site infection
In Figure 5, the meta-analysis of overall surgical site infections did not reveal a statistically significant difference between the two interventions (P = 0.56), but surgical site infections were more common in primary anastomosis than in Hartman’s procedure as indicated by the estimated overall effect size on the primary anastomosis side of the forest plot. There was limited heterogeneity with an I2 = 0%.
Surgical site infection for primary anastomosis and Hartman’s procedure performed for viable sigmoid volvulus
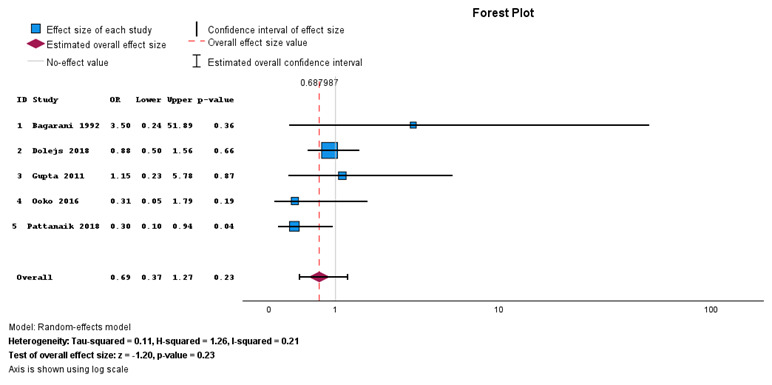
When comparing primary anastomosis and Hartman’s procedure for viable sigmoid volvulus, Figure 6 shows no statistically significant difference between the two interventions (P = 0.30), but primary anastomosis was associated with surgical site infection compared with Hartman’s procedure. There was no heterogeneity with I2 = 0%.
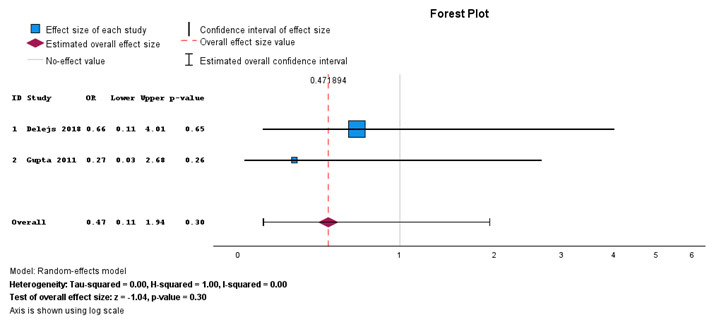
Surgical site infection for primary anastomosis and Hartman’s procedure performed for gangrenous sigmoid volvulus
When primary anastomosis is compared with Hartman’s procedure for gangrenous sigmoid volvulus, Figure 7 shows no statistically significant difference between the two interventions (P = 0.56). There is a substantia heterogeneity with I2 = 50%.
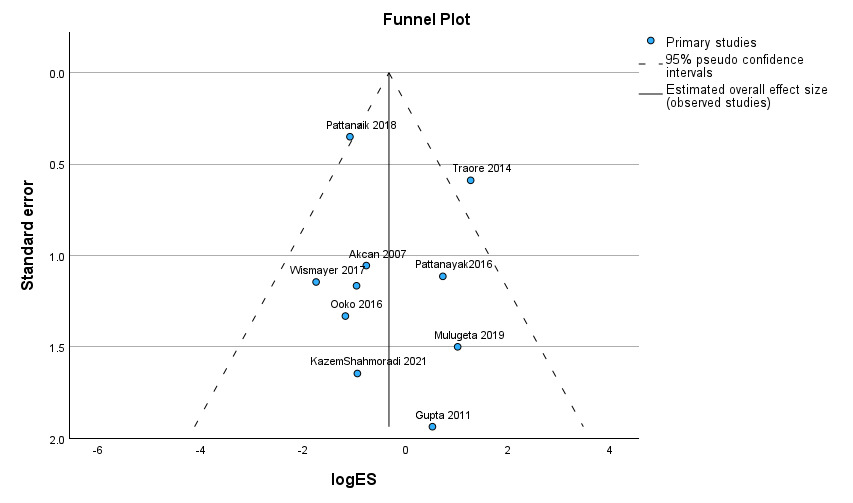
Publication Bias
Publication bias for primary outcomes (overall in-hospital mortality) was assessed using Egger’s regression-based test and supplemented with visual assessment via a funnel. Egger’s regression-based test with a P value of 0.516 revealed that there was no publication bias. A funnel plot is given in detail in Figure 8.
Discussion
Our study aims primarily to evaluate in-hospital outcomes between primary anastomosis and Hartmann’s procedure after sigmoid resection for sigmoid volvus, secondary to assessing other comorbidities or outcomes, as well as the demographics of patients with sigmoid volvulus.
In our study, 2853 (63.7%) males were predominated compared to 1620 (36.3%) females, which is similar to the findings of previous studies,8,11,16 as the male pelvis predisposes male patients to having a volvulus of the sigmoid compared to the female pelvis. The mean age in our study was 54.11 years, which is similar to other studies in which individuals above 50 years of age (40–70 years) were reported as patients with sigmoid volvulus.12,13,17 A study on the safety of primary anastomosis in gangrenous sigmoid volvulus by Riog reported a mean age of 27.5 years and a predominance of young individuals aged 21–30 years, which is different from our findings.18
In this study, viable sigmoid volvulus was more common 2911 (65.1%) compared to gangrenous sigmoid volvulus 904 (20.2%), while the remaining percentage was not reported in some studies. Our findings are similar to those of a study by Pattanayak, where the number of viable sigmoid volvulus cases was 79 (64.7%), which was similar to the 74.3% reported by Akcan,19 whereas the number of gangrene and perforation cases was 43 (35.3%).8 However, our findings are different from those of a study by Riogi, where the percentage of gangrenous sigmoid volvulus was 75%.18
In this study, overall in-hospital mortality was higher for primary anastomosis than for Hartmann’s procedure, but the difference was not statistically significant (P = 0.23). Similarly, a study by Akcan reported a mortality rate of 5 (5.5%) for primary anastomosis and 2 (4.4%) for Hartmann’s procedure, but this difference was not statistically significant (P = 0.82).19 A study by Gupta et al. reported that the mortality rate was 25% higher in the primary anastomosis group than in the Hartmann’s procedure group.20 A study by Ooko reported that mortality in primary anastomosis patients was low at 8.3%, whereas it was 22.7% for those who underwent Hartmann’s procedure.14
Our study revealed that there was no statistically significant difference (P value of 0.09 and 95% CI = 0.33–1.08) in mortality between the two procedures performed when sigmoid volvulus was viable, but higher mortality was noted in primary anastomosis than in Hartmann’s procedure. Several studies reported findings similar to those reported in our study.19,21,22
In our study, the mortality rate was greater with Hartman’s procedure than with primary anastomosis when sigmoid volvulus was gangrenous, but the difference was not statistically significant (P = 0.53). Patients who present with gangrenous sigmoid volvulus have a worse clinical picture than those with viable sigmoid volvulus. Several studies report poor outcomes when PRA is performed in patients with gangrenous sigmoid volvulus. Mulugeta et al. reported that 5 patients (6.5%) died after primary resection with anastomosis, but 3 of them were from gangrenous sigmoid volvulus. Our results are different from those of the study by Riogi, which reported the highest mortality rate of 33%, but these figures cannot be compared with the lowest mortality rates of primary anastomosis because few patients underwent resection and colostomy.18
The second outcome review in this systematic review was surgical site infection. Overall, surgical infection was most common in primary anastomosis, but this difference was not statistically significant (P = 0.22, 95% CI = 0.34–1.28). In a study by Chalya and Mabula, 13 (43.3%) surgical site infections were common with primary anastomosis, but no significant difference was noted between the two interventions.16 Akcan reported that surgical site infection was not significantly different between primary anastomosis (11, 12.1%) and Hartmann’s procedure (5, 11.1%) (P = 0.63).19
In this study, surgical site infection was more common in primary anastomosis than in Hartman’s procedure when there was a viable sigmoid volvulus, but there was no statistically significant difference (P = 0.34 and CI = 0.33–1.47). A study by Bhuiyan revealed that surgical site infection in primary anastomosis occurred in only 2 out of 39 cases (5.1%) and in Hartmann’s procedure occurred in only 1 out of 8 cases (12.5%). The results of this study differ from those of Gupta et al., who reported that surgical site infection was more common in Hartmann’s procedure with 5 out of 20 cases (25%) compared to primary anastomosis with 1 out of 12 cases (8.3%).20,21
Surgical site infection in gangrenous sigmoid volvulus patients in this study was more common with Hartmann’s procedure than with primary anastomosis, but the difference was not statistically significant (P = 0.86, CI = 0.15–9.96). In contrast to our study, Gupta et al. reported that the incidence of surgical site infection was in 6 out of 20 cases (30%) for primary anastomosis performed for gangrenous sigmoid volvulus and in 8 out of 30 cases (26.6%) for Hartmann’s procedure.20
No publication bias was noted in terms of overall mortality in either primary anastomosis or Hartmann’s procedure for sigmoid volvulus with Egger’s regression test being P = 0.399; similarly, analysis by Atalel et al. revealed no publication bias according to the funnel plot.23
Conclusion
Overall, primary anastomosis and Hartmann’s procedure showed no statistical difference in in-hospital mortality or surgical site infection. Clinical decision-making should prioritize patients’ stability, bowel viability, and resource availability.
Limitations
This study included few high-quality studies, such as randomized clinical trials, and the included studies were observational studies. Few studies address confounding factors for each procedure.
Ethical Approval
As the systemic review and meta-analysis didn’t involve direct involvement of human or animal subjects, ethical approval was not required.
Informed Consent
As the systemic review and meta-analysis didn’t involve direct involvement of human or animal subjects, ethical approval was not required.
Data Availability
Data that support the findings are available through a request to corresponding author.
Conflict of Interest
The authors report there are no conflicts of interest related to this work. No source of funds was given in this review.
Funding
No funds were given for this study to be carried out.
Acknowledgement
The authors gratefully acknowledge Ariel Pomptius, librarian at the University of Florida-USA, for expert guidance in developing comprehensive literature search strategies and valuable assistance during screening process.



_surgical_site_infect.png)



_surgical_site_infect.png)