Introduction
Surgical site infections (SSI) occur in a defined postoperative period following an operative procedure as an adverse event in surgical patient management, and result in high morbidity and mortality.1 The World Health Organization (WHO) has defined diagnostic criteria for SSI, classifying these infections as superficial SSI (infection develops within 30 days), deep SSI (infection develops within 90 days), and organ-specific or implant-associated SSI (infection develops within 1 year).2 WHO estimates that SSI are at 7.2% in the African region and that SSI are high in low- and middle-income countries like Zambia.3
SSI arise from hospital-acquired infections that reduce patient safety due to poor infection-prevention institutional practices.4 Reduction of patient safety is caused by the development of antimicrobial resistance microorganisms that add to the high cost of managing SSI and lead to increased hospital stays.5 In the perioperative period, adherence to guidelines can minimize the development of SSI, even though elimination of SSI is an immense challenge.3
SSI cause discomfort, increase patient hospital stays, raise the cost of management, and can result in catastrophic outcomes.6,7 The prevention of SSI requires a clean hospital environment, sterile operating room (OR), and the implementation of evidence-based SSI management protocols.3,8 Understanding SSI risk factors responsible for the development of SSI and formulating prevention strategies require institutional studies that elucidate data on prevailing microorganisms and their antimicrobial resistance patterns.8
This study aimed at determining the prevalence, risk factors for developing SSI, and antimicrobial resistance patterns at Ndola Teaching Hospital, Kitwe Teaching Hospital, and Arthur Davison Children’s Hospital, in the Copperbelt Province of Zambia.
Methodology
This was a prospective observational cross-sectional study conducted from January 2020 to December 2021. Convenient sampling of patients who were available for admission and fulfilled the inclusion criteria was used to recruit study participants. This study was conducted in the Copperbelt Province of Zambia at 3 tertiary hospitals. The Copperbelt Province has 3 tertiary public hospitals, namely Ndola Teaching Hospital, Kitwe Teaching Hospital, and Arthur Davison Children’s Hospital.
A total of 1122 participants were included in the study. This study primarily focused on superficial SSI, defined by WHO as postoperative infections occurring in the operative wound 30 days following a procedure. We followed the WHO Surgical Unit-based Safety Program, which advises that surveillance of SSI can be done with any number of patients. The study used data collection sheets that were modified from the WHO Surgical Unit-based Safety Program surveillance form, and data were entered into Microsoft Excel for cleaning and analysis.
Procedure
After confirmation of diagnosis, for surgical patients presenting to admission wards, by evaluation of clinical details, physical examination, and appropriate investigations, patients who underwent an operative surgical procedure that was superficial to deep fascia were enrolled per the inclusion and exclusion criteria. The presence of SSI was based on symptoms and signs of SSI, identification of the microorganism and its antimicrobial sensitivity, and resistance patterns. Symptoms included pain and pus draining from the wound site, while signs included redness, swelling, pus drainage, and wound dehiscence. Pus swabs collected were subjected to microscopy, culture, and sensitivity. Phone follow-up was also used with patient wound-site pictures. Informed consent was obtained from patients or guardians. Information was collected according to perioperative and postoperative forms and phone follow-up forms. Wound surveillance was conducted for 30 days from the time of the procedure.
Ethics
Ethics approval was obtained from the Tropical Diseases Research Centre Ethics Review Committee, with IRB registration number 00002911, and the National Health Research Authority.
Statistical Analysis
Using IBM SSPS Statistics, descriptive analysis was performed using proportions and frequencies for categorical variables, means with standard deviations, and medians with interquartile ranges for skewed data. Associations of variables were analyzed using the chi-square test for categorical variables. Further analysis of the outcome was conducted using logistical regression.
Results
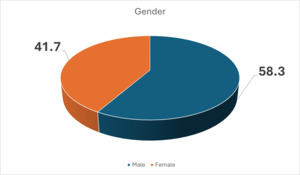
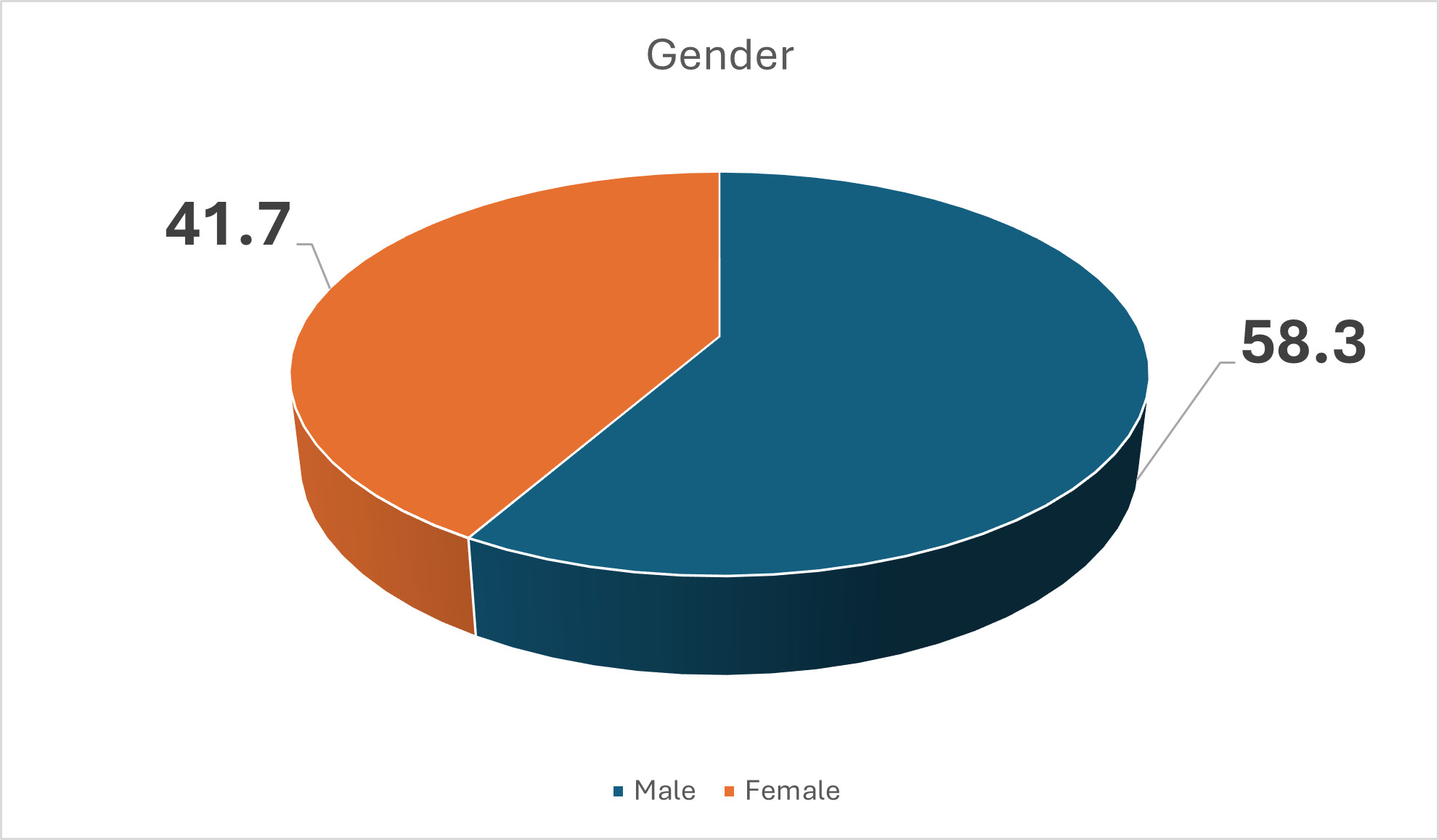
A total of 1122 participants were included. Of these, 468 were females and 654 were males. Figure 1 presents the baseline characteristics of patients enrolled in the study. Figure 1 also shows that more males than females developed SSI. However, gender was not a risk factor for SSI, with a P value of 0.319.
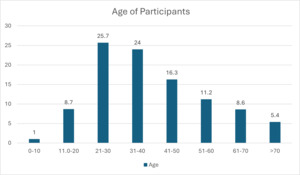
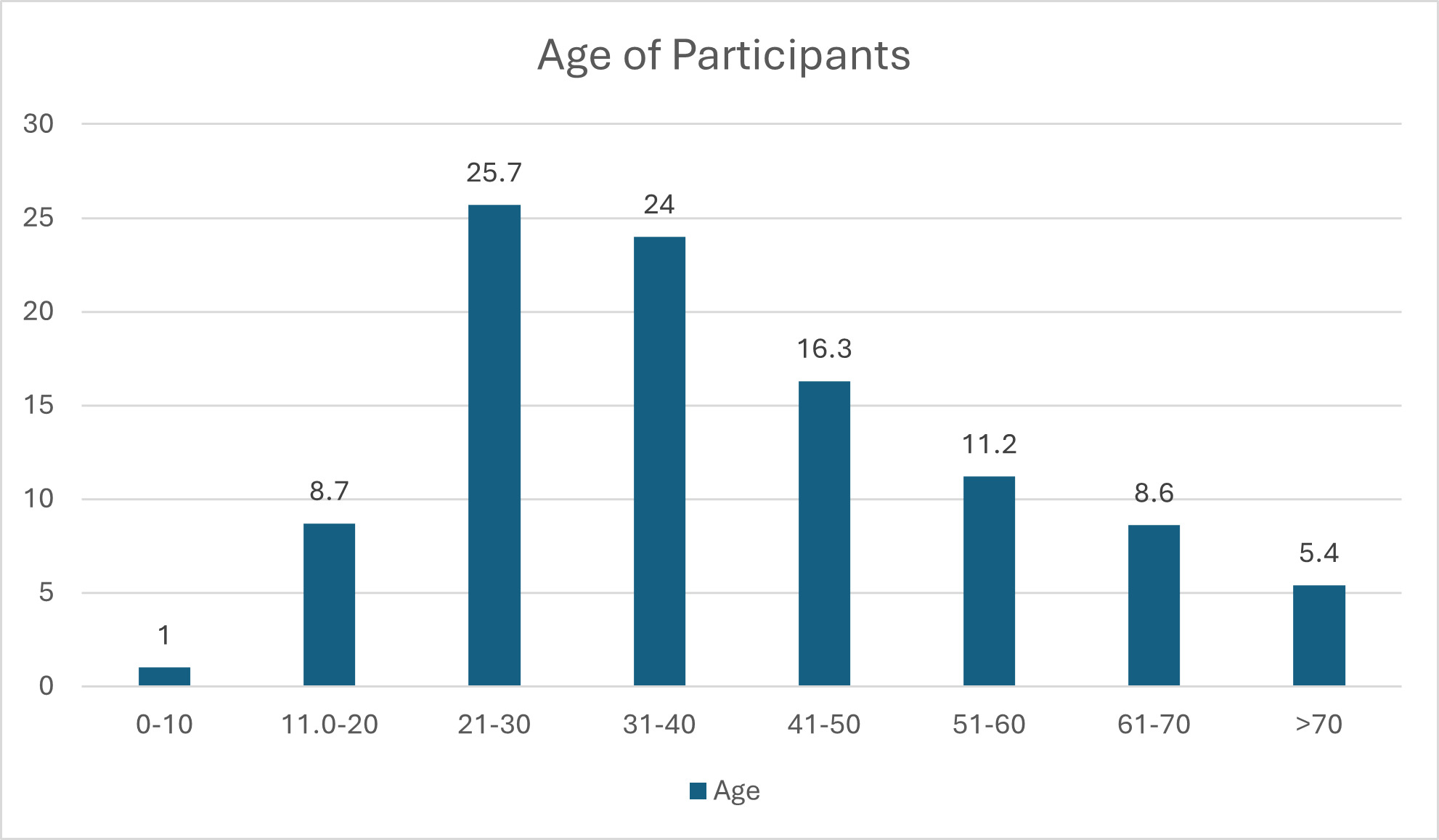
The results in Figure 2 show that the majority (25.7%) of the participants were aged between 21–30 years, followed by those between 31–40 years (24%), with the smallest number being those aged 10 years and below (0.1%). However, age was not significant for development of SSI, with a P value of 0.319.
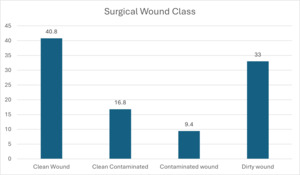
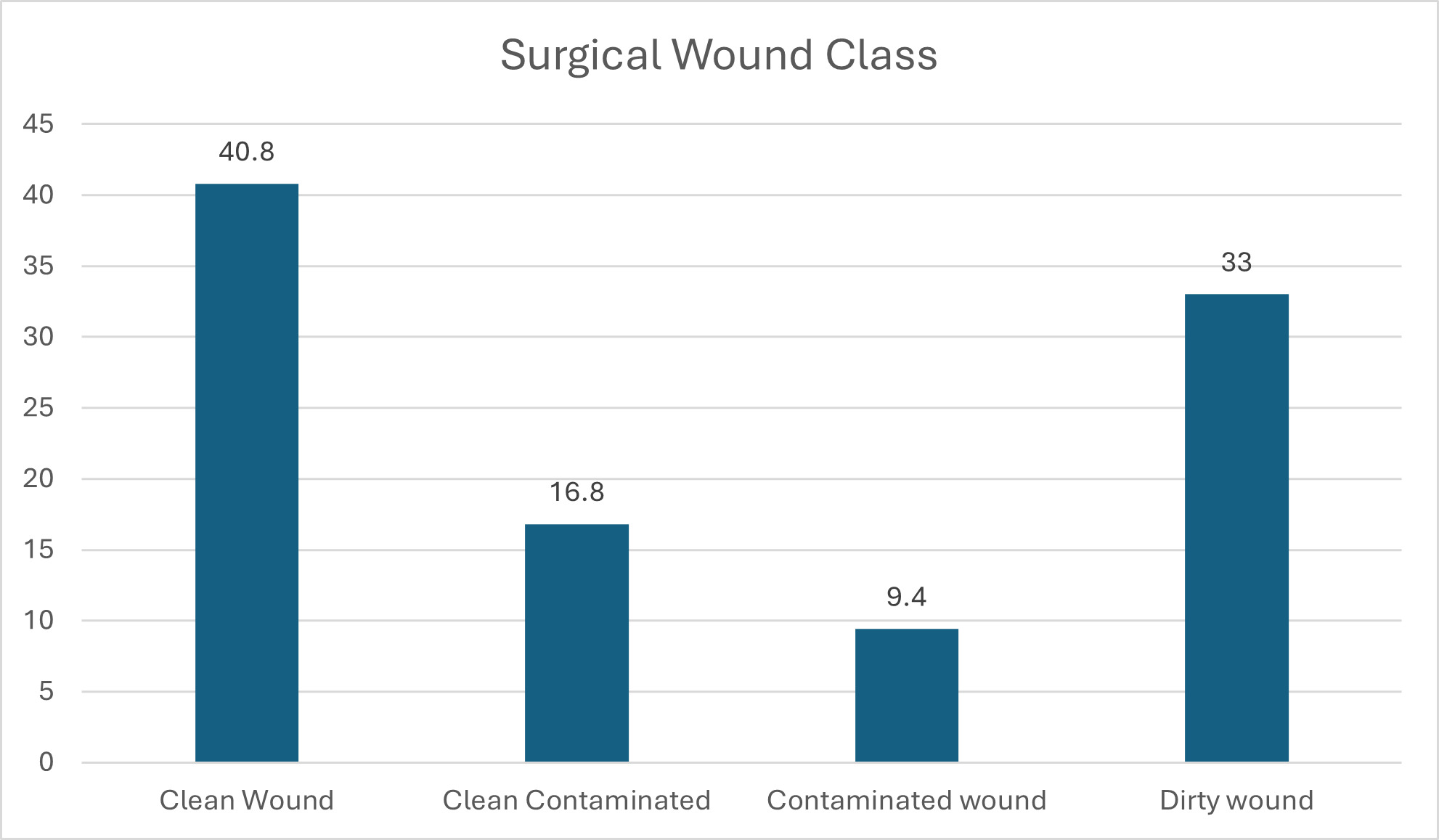
Figure 3 shows that most (40.8%) participants had clean wounds, followed by those with dirty wounds (33%). Contaminated and dirty wound categories consisted of patients who had infections present at time of surgery, representing 476 participants (42.4%). Wound class and development of SSI was not significant, with a P value of 0.921.
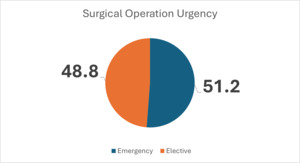
Figure 4 shows that the majority of participants had emergency surgery; however, the difference between the groups is minimal and not significantly related to development of SSI, with a P value of 0.235.
Table 1 shows the assessment of risk factors present in the OR environment, which were independently assessed for risk of developing SSI. The P values of less than 0.05 are significant for development of SSI, and these include the following risk factors for SSI: American Society of Anesthesiologists (ASA) score 3 and 4, long duration of surgery, not having a perioperative bath/shower, inappropriate skin preparation, not administering prophylactic antibiotics, presence of a drain, and presence of more than 15 people in addition to the operating team.
Figure 5 shows that a total of 218 participants (19.4%) whose pus swab samples were submitted developed SSI for analysis. A total of 904 participants (80.5) had no infection after initial surgical and antimicrobial treatment, of a total of 1122 participants.
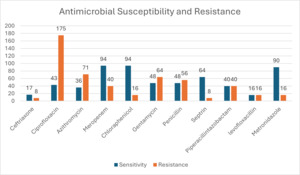
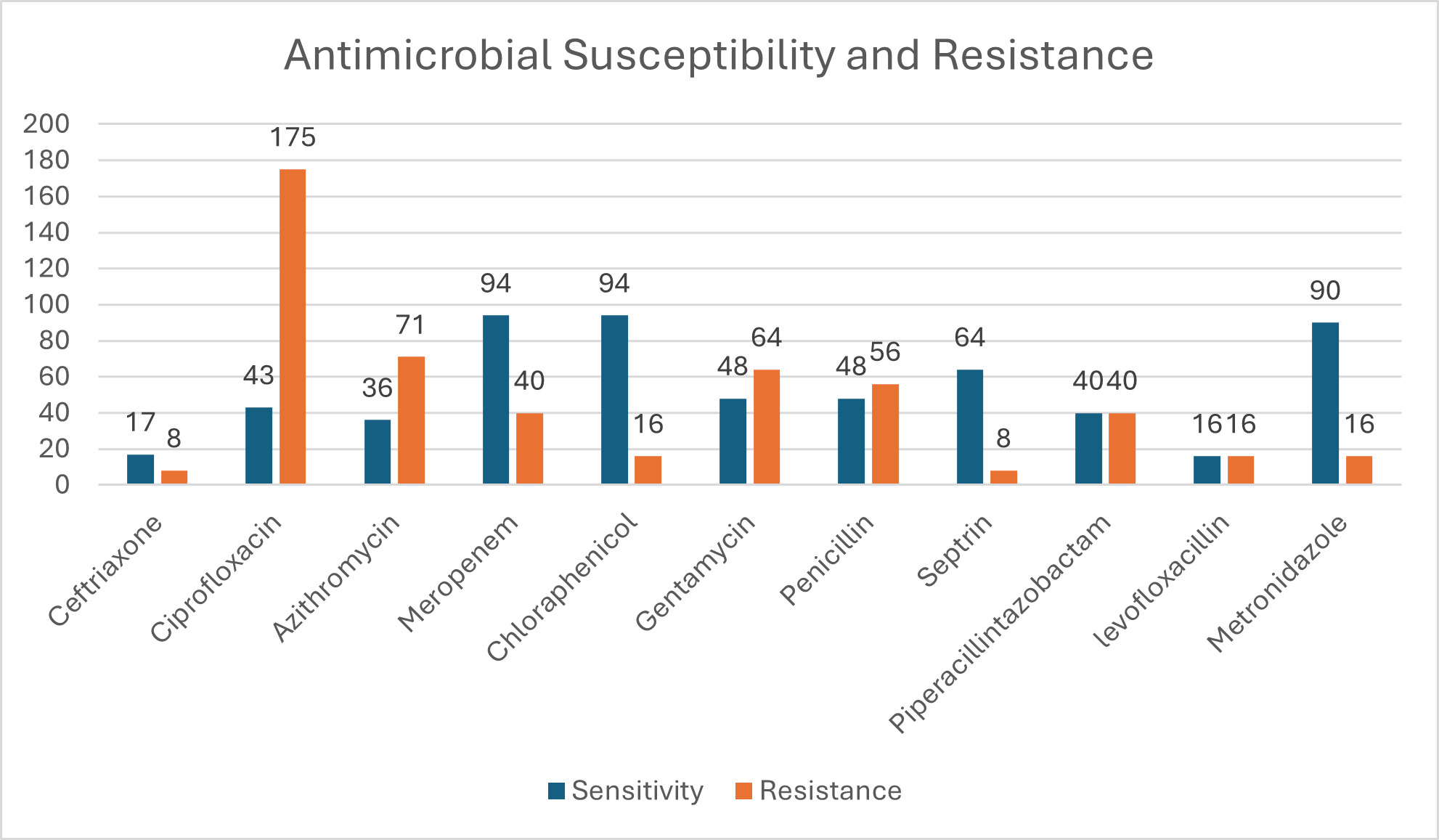
Figure 6 shows that a total of 482 participants (42.4%) presented with infection at the time of surgery, but after broad spectrum antimicrobial and surgical treatment, 264 of them (23%) responded well to treatment and did not develop SSI. Figure 6 represents susceptibility results of those who developed SSI and those who presented with infection at time of surgery. Ciprofloxacin had the highest resistance, followed by azithromycin and penicillin. The highest sensitivity was to ceftriaxone and chloramphenicol, followed by metronidazole.
Table 2 shows the resistance patterns of microorganisms that were cultured. Most bacteria were resistant to ciprofloxacin, metronidazole, and gentamycin.
Table 3 shows 3 multidrug resistant (MDR) species, namely Serratia marcescens, Yersinia pestis, and Corynebacterium species. This may also be a limitation of the antibiogram used to check sensitivities. More studies are required to evaluate these organisms.
Discussion
This study shows that the prevalence of SSI at the 3 tertiary hospitals in the Copperbelt Province of Zambia is 19.4%, as shown in in Figure 5. The patient population assessed included patients with infection already present at time of surgery (42.4%) and those who did not have infection and therefore had clean wounds (40.8%). The study population represents surgical referrals from first-level and second-level hospitals from all the districts of the Copperbelt Province in Zambia, including 5 other provinces in the northwestern half of Zambia. Hakayuwa et al9 found an SSI rate of 18% at Ndola Teaching Hospital Department of Surgery, Ndola, in the Copperbelt Province of Zambia. Bayardorj et al10 in Tanzania found an SSI rate of 10.9%. Gillespie et al,11 in their meta-analysis, found a global SSI rate of 11%. WHO estimates the African SSI rate to be 7.2%, which is high.3 Our study has shown the highest SSI in the sub-Saharan African region, from the available studies reviewed.
In this study, there were more males than females, with a P value of 0.319. The gender and age of participants in Figures 1 and 2 were also not significant for development of SSI. Other studies have reported similar findings.9,12 In this study, Figure 3 shows that all patients were recruited before medical or surgical treatment, such that 40.8% had clean wounds, and 42.4% had a wound infection already present at time of surgery. Surgical wound class in Figure 3, however, was not statistically related to development of SSI, with a P value of 0.921. Wand et al13 report an SSI rate of 7% for clean and clean contaminated wounds; however, Prada et al14 report that this rate can be significantly reduced to 4% with preoperative bath and hair removal, appropriate skin preparation, and preoperative antibiotic administration for clean and clean contaminated wounds. In this study, wound class in Figure 3 was not a risk for development of SSI, while having a preoperative bath, appropriate skin preparation before surgery, and administering antibiotic prophylaxis were significant in avoiding development of SSI, with P values shown in Table 1 for these and other risk factors.8
Table 1 shows that high ASA patient class of 3 and 4, longer duration of operation, prescribed duration of time spent by surgeon on surgical hand preparation, and appropriate technique for handwashing were all significantly related to patients developing SSI. Other studies have reported similar findings.15
In this study population, both emergency and elective surgical procedures were performed (Figure 4); however, this was not significantly related to development of SSI, with a P value of 0.235, contrary to what others have reported.16 Malnutrition and overcrowding in the OR have been reported to be risk factors for development of SSI,17 as shown in Table 1. However, in this study, BMI was not a risk, with a P value of 0.677, while overcrowding, defined by having more than 15 people in an operating room, was a significant risk, with a P value of 0.030 (Table 1).18,19
In this study, administering preoperative antibiotics was significant in preventing SSI, but postoperative antibiotic treatment was not significant. The majority (57.6%) of participants had no infection at time of surgery, and those who had an infection received preoperative antibiotics and surgical debridement. The evidence suggests that preoperative antibiotics and surgical debridement can reduce or eliminate postoperative antibiotic care.20,21 More studies are needed to validate our findings.
Table 2 and Figure 6 show the microorganisms cultured and their antimicrobial resistance in this study. The top 3 microorganisms were Staphylococcus aureus, Escherichia coli, and Klebsiella pneumoniae, as reported in other studies in Africa.22,23 Other studies indicate that the growth of S aureus and E coli suggest contamination by handling, if samples were collected in the OR.23,24 In this study, a few samples were collected in the OR for those who had an infection present at time of surgery. The majority, however, were collected during the 30-day follow-up, suggesting fewer chances of contamination.
Table 3 shows that ciprofloxacin, azithromycin, and penicillin were the top 3 antibiotics with highest resistance, while highest sensitivity was to ceftriaxone and chloramphenicol, as reported in other studies. These antibiotics are the most commonly prescribed by clinicians, and this finding is not surprising.25 We could not find a study for comparison in Zambia that has published SSI culture results. We believe this is the first study in Zambia to tabulate the culture of microorganisms and assess the susceptibility results of SSI in Zambia. Lubega et al26 reported similar sensitivities and resistance profiles of SSI in their study. The antibiotics showing most sensitivity were ciprofloxacin and ceftriaxone, while the most resistant were penicillin and Septrin.
Our study discovered MDR organisms that include S marcescens, Y pestis, Corynebacterium species, and Enterobacter agglomerans. These organisms require more studies to validate this finding.
Conclusion
The prevalence of SSI at the 3 tertiary hospitals in the Copperbelt Province of Zambia was 19.4%. Patients who presented to the hospitals requiring surgery with infection already present comprised 42.4% of the patient population assessed. The most common microorganisms were S aureus, E coli, and K pneumoniae. Most microorganisms were resistant to ciprofloxacin, azithromycin, and penicillin, while the highest sensitivity was to ceftriaxone and chloramphenicol. This study has discovered MDR organisms and suggests that this should be verified by further studies. The risk factors for superficial SSI identified by this study include ASA score, duration of surgery, perioperative bath/shower, skin preparation technique, prophylactic antibiotics, presence of a drain, and presence of more than 15 people in addition to the operating team.
Recommendations
Based on the evidence from this study, we recommend the following:
-
Ceftriaxone and chloramphenicol should be used for antibiotic prophylaxis and treatment of SSI at the tertiary hospitals in the Copperbelt Province,
-
Ciprofloxacin, penicillin, and azithromycin should be removed and the first line for treatment of SSI be prescribed based on susceptibility studies on SSI isolates at the tertiary hospitals in the Copperbelt Province,
-
S marcescens, Y pestis, Corynebacterium species, and E agglomerans have been identified as MDR organisms; more studies are required to validate this finding.
Limitations
The evidence presented in this study is that of a prospective cross-sectional study. We recommend a randomized cohort study to validate our findings.
Acknowledgments
We thank the management of Ndola Teaching and Kitwe Teaching Hospitals for their support.
Ethical Approval
Ethics approval was obtained from the Tropical Diseases Research Centre Ethics Review Committee, with IRB registration number 00002911, and the National Health Research Authority.
Conflict of Interest
None
Funding
None