Introduction
Acute abdomen in pregnancy presents diagnostic and therapeutic challenges due to anatomic changes, fetal radiation risks, and delayed intervention consequences.1 The incidence is 1 in 500–635 pregnancies, with diagnostic inaccuracy persisting despite medical advancements.1 Enteroatmospheric fistula (EAF), a severe and life-threatening postoperative complication associated with abdominal surgical interventions, involves fluid loss, malnutrition, and sepsis, with mortality up to 30%. Wound dehiscence arising from intraabdominal sepsis during pregnancy is an uncommon occurrence, and managing such intricate cases poses significant clinical challenges, necessitating collaborative decision-making between surgical and obstetric specialists.2
This case report adheres to the SCARE (Surgical Case Report) guidelines, which provide a structured framework to maintain methodological precision and clarity in documentation.3 While extensive literature exists on enterocutaneous fistulas, to the best of our knowledge, this represents the first documented case of EAF in pregnancy in environments with limited resources. This case highlights the significant challenges of managing an enterocutaneous fistula (EAF) in a rural Congolese health care facility lacking essential resources such as diagnostic imaging, parenteral nutrition, and specialized neonatal care. It underscores the necessity for adaptive strategies grounded in fundamental surgical principles. Accordingly, this report aims to: (1) detail the documented management of a rare case of EAF occurring during pregnancy within this resource-limited setting, (2) analyze the adaptive strategies employed, demonstrating their alignment with established surgical principles (SOWATS), and (3) explore the ethical dilemmas inherent in balancing maternal and fetal priorities when advanced care is unavailable.
Case Presentation
Initial presentation and diagnosis
A 33-year-old woman, gravida 9, para 7 (with 6 living children), presented to a rural health facility in Lukafu, Democratic Republic of Congo, at 20 weeks’ gestation. She reported a 2-week history of fever treated with unspecified medications at home, followed by 3 days of progressive symptoms of gastrointestinal distress, including epigastric discomfort, recurrent emesis, persistent nausea, and a complete absence of bowel movements. On admission, physical examination revealed tachycardia (108 bpm), abdominal distension, involuntary guarding, and shifting dullness. Abdominal ultrasound confirmed intraabdominal free fluid and a viable intrauterine pregnancy. A clinical diagnosis of acute peritonitis secondary to intestinal perforation was made.
Management
Preoperative management included intravenous crystalloid resuscitation, administration of antimicrobial therapy (ceftriaxone, 2 g once daily, and metronidazole, 500 mg every 8 hours), proton pump inhibitors (pantoprazole, 40 mg IV once daily), nasogastric decompression, and urinary catheterization. After obtaining informed consent, an emergency midline exploratory laparotomy was performed. Intraoperative findings included fecal peritonitis, edematous bowel adhesions, and a single 4 x 5 cm perforation in the distal ileum located 20 cm proximal to the ileocecal junction. Segmental resection of the affected ileum with immediate restoration of bowel continuity was performed, followed by copious peritoneal lavage using 6 liters of normal saline. The abdomen was closed with interrupted sutures, and a pelvic drain was placed.
Postoperative course, complications, and management
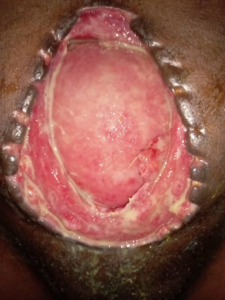
The early postoperative course was initially uneventful. The nasogastric tube and pelvic drain were removed on postoperative day (POD) 3, and oral feeding resumed. Antibiotics were continued for 7 days. On POD 7, the surgical wound exhibited signs of infection, including erythema, purulent discharge, and leakage of enteric contents. By POD 10, complete fascial dehiscence occurred, resulting in an open abdomen with a high-output entero-atmospheric fistula (3,000 mL/day) and exposed gravid uterus (Figure 1).
Conservative management was initiated due to limited resources. A low-fiber diet and loperamide (4 mg orally four times daily) were administered to reduce intestinal motility and fistula output. In the absence of laboratory capacity for electrolyte monitoring, hydration status was assessed clinically via urine output, skin turgor, and vital signs. Empiric potassium supplementation (0.5 mEq/kg/day IV) was maintained with adjustments based on clinical indicators of dehydration or cardiac rhythm irregularities. Daily wound care involved saline-soaked gauze dressings coated with petroleum jelly to protect peri-fistula skin from caustic effluent. Over subsequent weeks, fistula output gradually decreased to 300–500 mL/day with progressive granulation tissue formation around the wound edges.
Obstetric complications and fistula closure
Obstetric complications emerged at 25 weeks’ gestation (POD 35) when the patient reported uterine contractions. Physical examination revealed cervical softening, prompting tocolysis with hyoscine and rectal salbutamol. Contractions temporarily subsided but recurred at 28 weeks’ gestation (POD 56), culminating in spontaneous vaginal delivery of a live 1,200 g male infant. The neonate exhibited severe respiratory distress secondary to hyaline membrane disease and died within 24 hours due to the absence of neonatal intensive care.
Three months postoperatively (POD 90), the fistula closed spontaneously with near-complete wound epithelialization (Figure 2). Secondary skin closure was performed under local anesthesia, and the patient was discharged with instructions for outpatient follow-up.
Discussion
Bowel perforation during pregnancy, though uncommon, represents a critical surgical emergency demanding prompt intervention due to its high morbidity and mortality risks.
Evaluating acute abdominal pathology in gravid individuals presents unique challenges due to the expected anatomical, functional, immunological, and hematological adaptations of pregnancy, alongside efforts to minimize fetal radiation exposure during diagnostic imaging. Delayed diagnoses and treatment may have more serious consequences for pregnant women (and the fetus) than for other patients.4
Imaging studies can play a pivotal role in diagnostic evaluation, though clinicians often exhibit caution when employing these techniques due to potential risks to fetal development. Current recommendations emphasize maintaining cumulative fetal radiation exposure below the threshold of 5–10 rads during pregnancy to minimize teratogenic effects.5 Radiation exposure early in fetal development is particularly detrimental as critical organ development is taking place.
Fetal exposure levels from standard abdominal radiography typically range between 0.1 and 0.3 rads, whereas abdominopelvic CT imaging may deliver significantly higher doses, approaching 5 rads of ionizing radiation.6 High levels of ionizing radiation have been associated with genetic alterations, neurodevelopmental deficits, cognitive impairments, and an elevated likelihood of pediatric leukemia. Total radiation dosage over time serves as the critical determinant of harmful outcomes on fetal development; however, the timing of exposure during gestation plays a significant role.7,8
While uncomplicated typhoid fever during pregnancy may pose limited direct risks to fetal health, the development of typhoid-related intestinal perforation endangers both maternal and fetal survival. Pregnancy loss rates are markedly elevated, particularly if intestinal perforation complicates the infection during early and mid-gestational periods.9 Recent research involving a cohort of 80 gravid individuals diagnosed with uncomplicated typhoid infection and 194 gestation-matched controls without the infection found no significant differences in obstetric outcomes.10 Conversely, another series revealed that gestational status accounted for 9.3% of reported intestinal perforation cases, with mortality rates reaching 50% among affected pregnant patients.9
Following confirmation of intestinal perforation, expedited surgical intervention is imperative to optimize outcomes. While concerns regarding perioperative and anesthetic risks in pregnancy have prompted a shift toward nonoperative strategies in gravid patients, such approaches remain controversial. Published reports describe limited instances of conservative management for intestinal perforation, including two cases treated medically with antibiotic therapy, pain management, anti-inflammatory agents, and close monitoring.11,12 In both instances, however, the pregnancies ended in fetal demise. Notably, the case reported by Meshkov et al culminated in maternal mortality as well.11 In a third case reported, deferral of surgical intervention to address an obstetric complication culminated in fetal demise.13
Evidence highlights that timely surgical intervention in cases of intestinal perforation significantly impacts survival outcomes in non-pregnant populations.14 For instance, the standard of care for typhoid-induced perforations involves prompt surgical intervention combined with antimicrobial therapy, ideally initiated within six hours of initiating adequate resuscitation.15 Emerging evidence supports the use of image-guided percutaneous drainage as an effective modality for managing intra-abdominal abscesses across diverse etiologies.16 Among these studies, one clinical series demonstrated a 91.6% success rate in achieving resolution.17 This is only utilized when there has been a delay in diagnosis allowing an abscess to form. There are no clear guidelines advocating for this approach in pregnant patients.
The literature seems to support early laparotomy for maternal and infant survival.18
A generous abdominal incision is essential to ensure sufficient surgical access while reducing uterine handling. The approach to the pregnant uterus is guided by gestational age. Cesarean delivery might be required to facilitate adhesiolysis when surgical access is restricted due to systemic infection or peritonitis, particularly if fetal viability necessitates expedited delivery. Vaginal birth is favored when feasible to expedite, given the risks of infection associated with uterine incision in the context of intestinal perforation.19
In the case we present above, the patient was adequately rehydrated and received broad spectrum antibiotic therapy before undergoing a prompt abdominal exploration which made it possible to identify the perforation and treat it. It did allow for a delayed vaginal delivery, since the viability of the fetus in our setting was an issue. Despite resource constraints, antenatal corticosteroids (e.g., betamethasone) could have been administered when preterm labor was identified at 25 weeks. Evidence indicates corticosteroids reduce neonatal mortality from hyaline membrane disease by 40% in low-resource settings,20 and their absence here highlights a modifiable gap. Stocking low-cost corticosteroids should be prioritized in rural facilities
Enterocutaneous fistula (ECF) rank among the most challenging postoperative complications in gastrointestinal surgery. A newly categorized subtype, entero-atmospheric fistula (EAF), arises in the context of an open abdominal cavity, where the fistulous opening lacks surrounding epithelialized tissue.21 As described by Marinis et al, EAF is characterized by the absence of a defined fistulous tract and is often associated with etiologies such as anastomotic dehiscence, the use of temporary abdominal closure techniques, inflammatory adhesions between the bowel and abdominal wall, postoperative infections, abdominal wound dehiscence, and intestinal ischemic events.
The formation of a fistula can induce inflammation within the peritoneal cavity and lead to dense adhesion formation, increasing the risks associated with immediate surgical intervention.22 These adverse intra-abdominal conditions, often linked to open abdominal wounds, may persist for up to two months following initial injury or exposure.23 Furthermore, research indicates that mortality rates related to surgical procedures rise markedly when operations are conducted between 11 and 42 days after fistula onset.22
The principles of treatment for enterocutaneous fistula are: immediate control of sepsis if present, nutrition optimisation, fluid and electrolyte support in the form of parenteral nutrition (PN), wound/effluent control, and protection of surrounding tissues and exposed bowel.24
Multiple techniques exist to separate an entero-atmospheric fistula (EAF) from adjacent intestinal structures or healing tissue. Utilizing topical negative pressure therapy (NPWT) can promote tissue repair and manage fluid drainage. This method shields nearby tissue, improving secure wound coverage and facilitating later surgical interventions, while also reducing injury to nearby organs and lowering the likelihood of recurrent fistulas.24–27
In our case, in the absence of NPWT, we used conventional dressing changes, a low-fiber diet and bowel-slowing medications. These measures allowed a decrease in fistula drainage and minimized peristomal skin damage, ultimately resulting in spontaneous resolution of the fistula without surgical intervention.
Ethical tensions arose in balancing maternal stabilization against fetal survival. While transfer to a tertiary center (300 km distant) was theoretically possible, impassable roads during rainy seasons and lack of ambulance services rendered this impractical. This reflects systemic inequities requiring policy-level solutions: decentralized neonatal care units, maternity transport networks, and telehealth consultations for complex cases.28
This study has several limitations: (1) as a single-case observation, findings lack generalizability, (2) no literature comparison group exists for EAF in pregnancy, (3) retrospective design precludes standardized outcome tracking, and (4) long-term maternal outcomes (e.g., fertility, adhesive bowel obstruction) remain unassessed due to lost follow-up.
Conclusion
Conservative EAF management achieved maternal survival, but neonatal death highlighted systemic gaps. In similar settings, we recommend: (1) protocolized antenatal corticosteroids for preterm labor, (2) district-level partnerships for emergency transfers, and (3) training in enterostomal therapy. Future guidelines must integrate obstetrical-surgical coordination, contextual ethics frameworks, and investment in perinatal infrastructure to optimize maternal-fetal outcomes
Ethical Approval/Informed Consent
Formal ethical approval was not required for this case report as per institutional guidelines/local regulations for retrospective reporting of a single clinical case. The patient provided written informed consent to publish this case report and associated images. They acknowledged that all identifying details would be anonymized to ensure privacy, and that the content and visuals might be disseminated publicly. The signed consent documentation is accessible upon request.
Data Availability
Not applicable
Conflicts of Interest
The authors affirm that no competing interests, financial or non-financial, exist associated with the development or dissemination of this work.
Funding
No external funding was secured for this study from public, private, or nonprofit organizations.
_90._*.png)

_90._*.png)